Mitochondria, crucial organelles for metabolite and energy production, play a significant role in age-related processes, including cancer and neurodegeneration. Recent research has highlighted the potential importance of mitochondrial fragmentation in the development of various neurodegenerative diseases, such as Alzheimer's, Huntington's, and Parkinson's. Numerous studies have observed that prolonged mitochondrial fragmentation results in increased heterogeneity among mitochondria. However, the mechanisms proposed to explain these observations may not be entirely persuasive or conclusive.
In a new study, a research team led by Tatsuhisa TSUBOI at Tsinghua Shenzhen International Graduate School (Tsinghua SIGS) and coauthors, has shed light on this mystery. They proposed a novel, holistic explanation for mitochondrial dysfunction: “co-translational protein targeting, where the proteins are synthesized and transported to mitochondria simultaneously, amplifies protein heterogeneity across mitochondrial fragments, ultimately leading to mitochondrial dysfunction during cell senescence.”
In this study, the researchers measured the heterogeneity (noise) in the distribution of nuclear-encoded proteins between yeast mitochondrial fragments using advanced microscopy techniques coupled with comprehensive computational analysis. Their findings provide a new understanding of the physical changes associated with mitochondrial fragmentation, leading to heterogeneity in protein distribution. They observed that nuclear-encoded mRNA localization and co-translational protein import contribute to the generation of heterogeneity by restricting protein production to specific mitochondrial fragments using quantitative microscopy methods. Specifically, they reported that smaller mitochondrial fragments are associated with increased variability in protein expression levels (Figure 1).
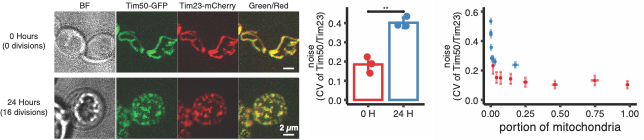
Figure 1: Aging-induced mitochondrial fragmentation increase the protein expression heterogeneity
Mitochondrial dysfunction is a hallmark of aging. Mitochondria require a precise complement of proteins to fulfill their diverse functions within the cell, however, encode only a small subset of proteins. The majority are nuclear-encoded and require intricate post-synthesis delivery to mitochondria, a process crucial for maintaining mitochondrial health and cellular homeostasis. Using computational modeling, the researchers demonstrated that co-translational protein delivery, where proteins are synthesized and inserted into mitochondria simultaneously, plays a key role in amplifying this heterogeneity (Figure 2). Subsequently, the researchers experimentally validated the role of co-translational protein targeting in driving protein expression heterogeneity.
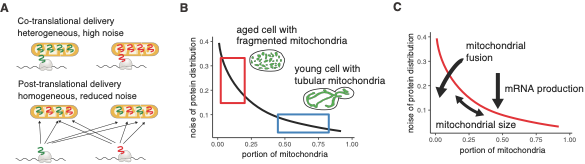
Figure 2: Mitochondrial fragmentation cause heterogeneity in protein distribution via mRNA localization and co-translational protein delivery
The study reveals that mitochondrial dysfunction arises not only from mtDNA mutations or mitophagy related issues but also from co-translational protein targeting. Their ongoing work investigates the complex interplay between mitochondrial fragmentation, co-translational protein import, and proteostasis. The team is exploring genetic regulatory mechanisms governing co-translational protein import and searching for potential inhibitors. Given the established link between mitochondrial dysfunction and various diseases and aging, they are now examining the correlation between mitochondrial fragmentation and protein heterogeneity in human cells and animal models. This avenue of investigation could lead to novel therapies targeting mitochondrial protein production, potentially preventing or slowing the progression of mitochondria-related diseases and age-associated disorders.
The study was published in Nature Communications under the title “Mitochondrial protein heterogeneity stems from the stochastic nature of co-translational protein targeting in cell senescence.” The corresponding authors of this article are Tatsuhisa TSUBOI, Assistant Professor at Tsinghua SIGS, and Brian M. ZID, Professor at University of California, San Diego. The first author is Abdul Haseeb KHAN, postdoc researcher at Tsinghua SIGS. Other research collaborators include GU Xuefang, master’s student at Tsinghua SIGS, Rutvik J. PATEL and Prabha CHUPHAL, respectively undergraduate student and postdoc researcher at Toronto Metropolitan University, Matheus P. VIANA, Senior Scientist at Allen Institute for Cell Science, and Aidan I. BROWN, Assitant Professor at Toronto Metropolitan University. This work is supported by the National Key Research and Development Program of China, the Science, Technology, and Innovation Commission of Shenzhen Municipality, startup funds and Interdisciplinary Research and Innovation Fund from Tsinghua SIGS.
Link to full article:
https://doi.org/10.1038/s41467-024-52183-y
Written/Figures by Abdul Haseeb KHAN, Tatsuhisa TSUBOI
Edited by Huang Xiaojia
Reviewed by Chen Chaoqun


