The overuse of fossil fuels has caused serious energy and environmental problems. Developing alternatives to fossil fuels and achieving carbon neutrality have become common goals of global society. As a zero-carbon emission energy with high energy density, hydrogen energy has been considered as one of the most ideal energy sources for the future due to its high energy density and zero-carbon emission character. Water electrolysis is an important technology for green hydrogen production, but its practical application is hindered by its high energy consumption and catalyst cost. Therefore, reducing the energy consumption and equipment costs of hydrogen production by developing electrocatalysts with high performance and low cost is the key to achieving large-scale production of green hydrogen.
Ni-based catalysts are promising for alkaline hydrogen evolution reaction (HER) because of their high catalytic activity, high electrical conductivity, and low price. However, their performance at high-current densities needs to be improved to fulfill the demands of practical industrial application. Recently, the team led by Associate Prof. Bilu Liu from Shenzhen International Graduate School (SIGS) and cooperating researchers developed a nickel-based catalyst (h-NiMoFe) that exhibits excellent performance under high-current densities and clarified its catalytic mechanism by in-situ spectroscopic characterization and density functional theory (DFT) calculations.
Researchers prepared the h-NiMoFe catalyst using a two-step method of hydrothermal reaction combined with high-temperature thermal reduction. First, the catalyst has a three-dimensional porous structure, which ensures adequate contact between catalysts and electrolytes under high-current densities. Second, using in-situ X-ray absorption spectroscopy and quasi-in-situ X-ray photoelectron spectroscopy, researchers found that the molybdenum and iron in the catalyst significantly tuned the charge distribution of nickel sites and increased the valence state of nickel. Third, the surface chemistry of the catalyst was also changed. The nickel-based catalyst modified by molybdenum and iron simultaneously shows a highly hydroxylated surface. DFT calculation further shows that the iron species changed the position of the d-band center of the nickel site and optimized the free energy of hydrogen adsorption. Meanwhile, the water dissociation steps of the alkaline hydrogen evolution reaction have also been optimized. The aforementioned micro-nano structure, charge distribution on active sites, and surface chemistry of catalyst together endow h-NiMoFe catalysts with excellent hydrogen evolution performance at high-current densities.

Fig. 1 Synthesis and characterization of h-NiMoFe catalysts

Fig. 2 X-ray absorption spectroscopies of h-NiMoFe catalysts

Fig. 3 X-ray photoelectron spectroscopies of h-NiMoFe catalysts before and after reaction
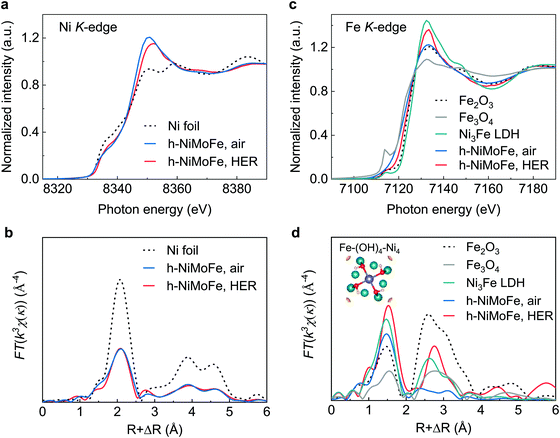
Fig. 4 In-situ X-ray absorption spectroscopies of h-NiMoFe catalysts

Fig. 5 DFT calculation results
The h-NiMoFe catalysts only require a low overpotential of 97 mV to reach a high-current density of 1000 mA cm-2, which is better than most reported catalysts. This catalyst was used in overall water splitting, which achieved a current density of 500 mA cm‒2 at a record-low cell voltage of 1.56 V and realized a stable operation under high-current densities. The h-NiMoFe catalyst has a low price of ~82 US$ m-2 that is close in price to commercial RANEY® Ni catalysts, but its performance is much better than RANEY® Ni catalysts, indicating its potential in practical application. This study provides a new perspective for the development of low-cost hydrogen evolution catalysts; the strategy of improving catalyst performance through charge regulation is expected to be further extended to other materials and reactions.

Fig. 6 High-current densities hydrogen evolution performance

Fig. 7 Scalable synthesis of FeMC-NiMo and overall water splitting
These results have recently been published in the journal Energy & Environmental Science in a paper titled "Stabilized Hydroxide-Mediated Nickel-Based Electrocatalysts for High-Current-Density Hydrogen Evolution in Alkaline Media." The corresponding authors are Prof. Bilu Liu and Prof. Shuo Zhang. The first author is Yuting Luo; Zhiyuan Zhang is the co-first author. The authors of this paper also include Fengning Yang, Prof. Li Jiong, Prof. Ren Wencai, and Dr. Zhibo Liu. This work was supported by the National Natural Science Foundation of China, the Guangdong Innovative and Entrepreneurial Research Team Program, the Science, Technology and Innovation Commission of Shenzhen Municipality, the Development and Reform Commission of Shenzhen Municipality, and the Industry and Information Technology Bureau of Shenzhen Municipality.
Link to full article:https://pubs.rsc.org/en/content/articlelanding/2021/ee/d1ee01487k
Written by Zhang Zhiyuan
Edited by Alena Shish, Yuan Yang


