A high-throughput and automated organoid platform developed by Tsinghua-Berkeley Shenzhen Institute (TBSI) researchers could speed up and address challenges in organoid production.
The breakthrough was recently published in the journal Cell Reports Medicine entitled “An Automated Organoid Platform with Inter-organoid Homogeneity and Inter-patient Heterogeneity” by the Shaohua Ma & Laiqiang Huang research team at the Precision Medicine and Healthcare Research Center, TBSI.
Organoid is an in vitro three-dimensional (3D) cell culture technology that captures and stably passes down the genomic and phenotypic profiles of human healthy organs and tumors. They are scalable, easy-to-culture, and may evaluate patient tumor sensitivity to anticancer drugs. However, current organoid technologies are limited by significant variability, labor-intensive techniques, long culturing times, and tumor organoids in particular face the challenge of maintaining intra-tumor and inter-patient heterogeneity.
The paper presented an automated organoid platform that generates uniform organoid precursors in high-throughput. This is achieved by templating from monodisperse Matrigel droplets and sequentially delivering them into wells using a synchronized microfluidic droplet printer. Each droplet encapsulates a certain number of cells (e.g. 1500 cells) which statistically represent the heterogeneous cell population in a tumor section. The system produces > 400-μm organoids within 1 week (as opposed to 4-6 weeks of culturing time) with both inter-organoid homogeneity and inter-patient heterogeneity. Using this technology platform, the team has successfully produced organoids from various healthy tissues and tumors of mouse and human organs.
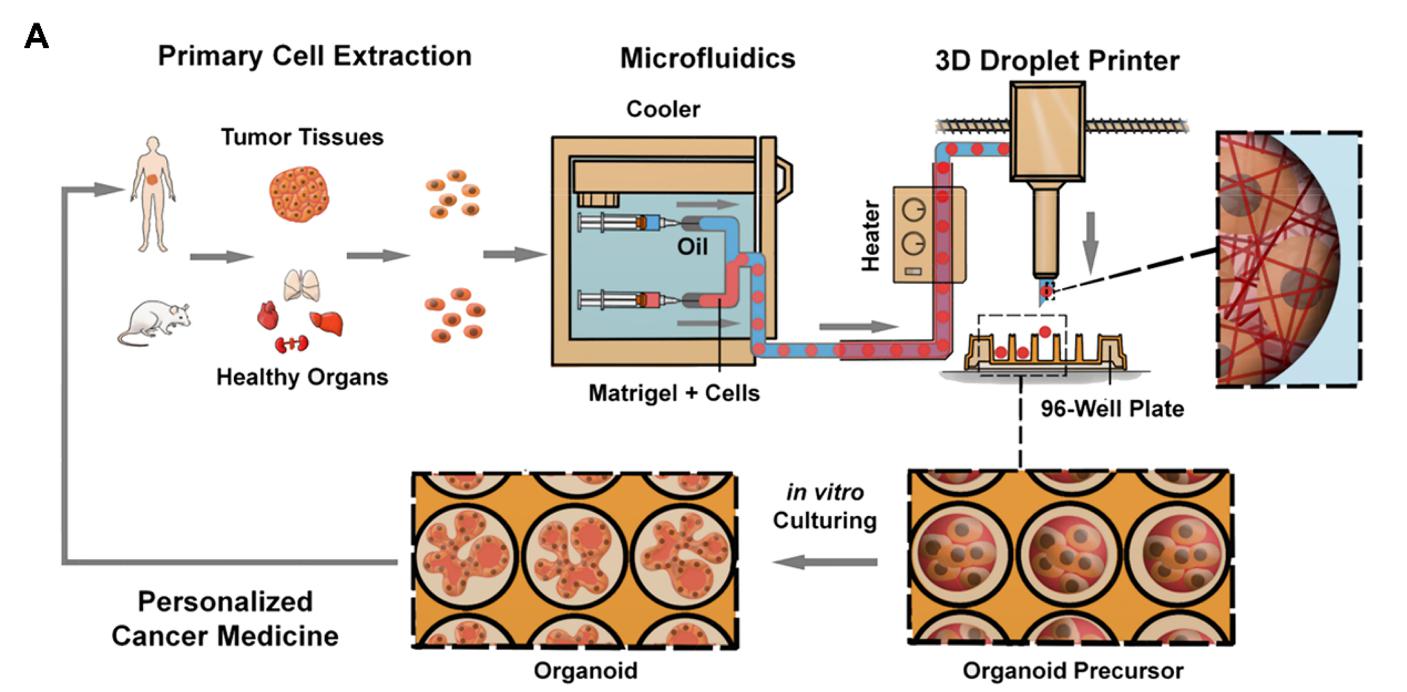
Figure 1.The automated organoid platform
To assess whether the organoids resembled characteristics of their parental healthy tissues and tumors, histological analysis was carried out. Results showed that mouse organoids derived from lung, kidney and liver displayed similar histology to the original cell, with significant recapitulation of epithelial organization. Contrarily, human tumor organoids displayed much reduced epithelium in volume. In terms of gene expression, tumor-derived organoids were found to have a highly overlapped (over 97%) single nucleotide variants (SNV) profile with their parental tumor tissue.
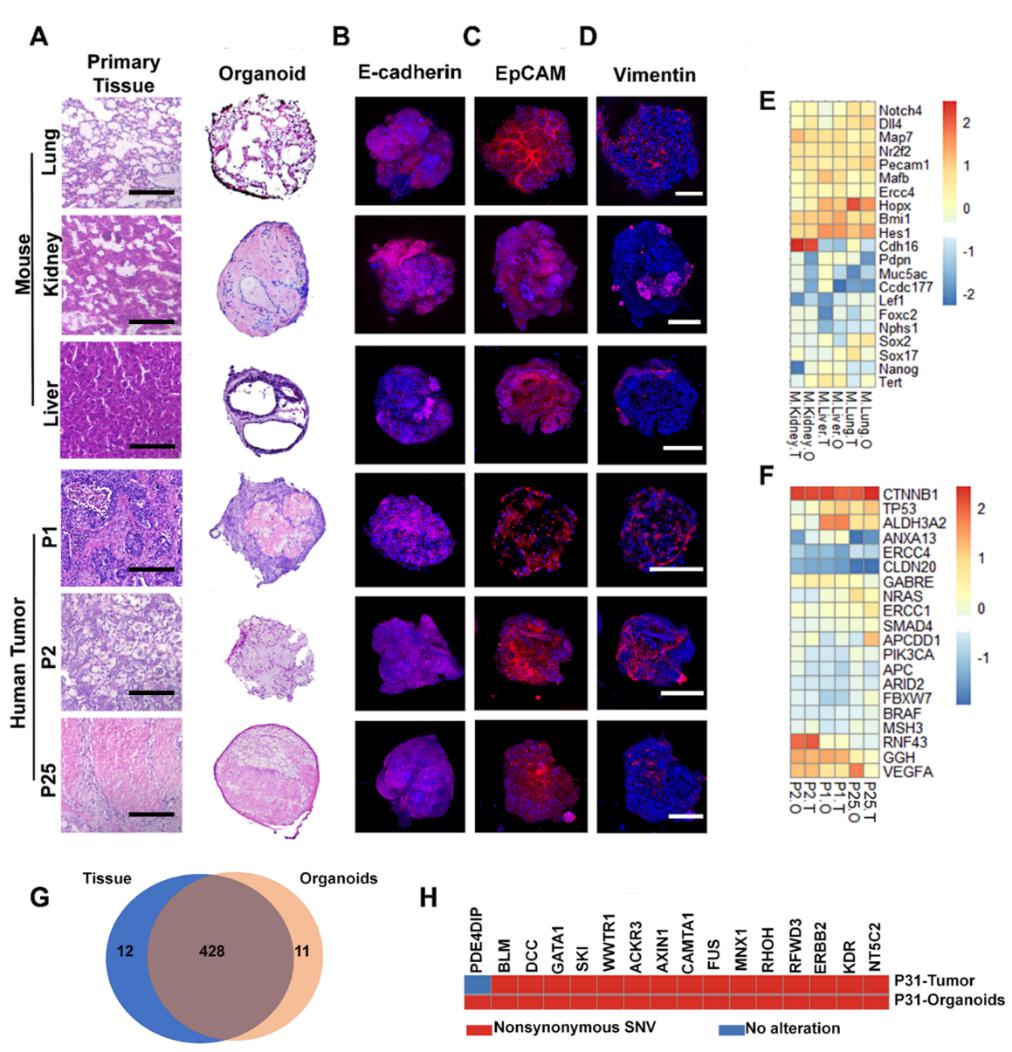
Figure 2. Histopathological characterization and gene expression profiling of healthy and tumor organoids
The team also assessed the potential of the organoids in evaluating different drug responses. Therapeutic profiling on an anticancer drug library with 29 chemotherapeutic drugs and 2 targeted drugs, rituximab and cetuximab, showed 80% accuracy in 21 patients.

Figure 3. Tumor organoids capture inter-patient heterogeneous responses to anticancer drugs
Capable of creating multi-organ, cross-species, healthy and cancerous organoids in high throughput, high uniformity and reduced manual manipulation, the developed platform is expected to play increasing roles in personalized cancer medicine, and theranostics innovation for new drug development, and regenerative medicine.
For this article, TBSI Professor Laiqiang Huang and Associate Professor Shaohua Ma are the corresponding authors, and their respective TBSI Ph.D. students Shengwei Jiang and Haoran Zhao are the first authors.
Link to the article:
https://www.cell.com/cell-reports-medicine/fulltext/S2666-3791(20)30208-1
Written by Shengwei Jiang
Edited by Karen Lee


